Progress towards a cure for Rett Syndrome by RNA editing.
Introduction. I have previously written articles which appear on the RSAA website that describe strategies designed to cure Rett Syndrome; these strategies, listed below, are designed to result in the expression of functional MeCP2 protein in brain cells of individuals with Rett Syndrome.
- Gene therapy
- DNA editing
- RNA modification and RNA editing
- RNA trans-splicing
- MeCP2 reactivation
- MeCP2 protein replacement
Several of these strategies depend on the Central Dogma of molecular biology of the cell ie. that genetic information flows in one direction, from DNA (contained in the genome) to messenger RNA (mRNA) to protein (Figure 1).
Figure 1 with acknowledgement to the Khan Academy.
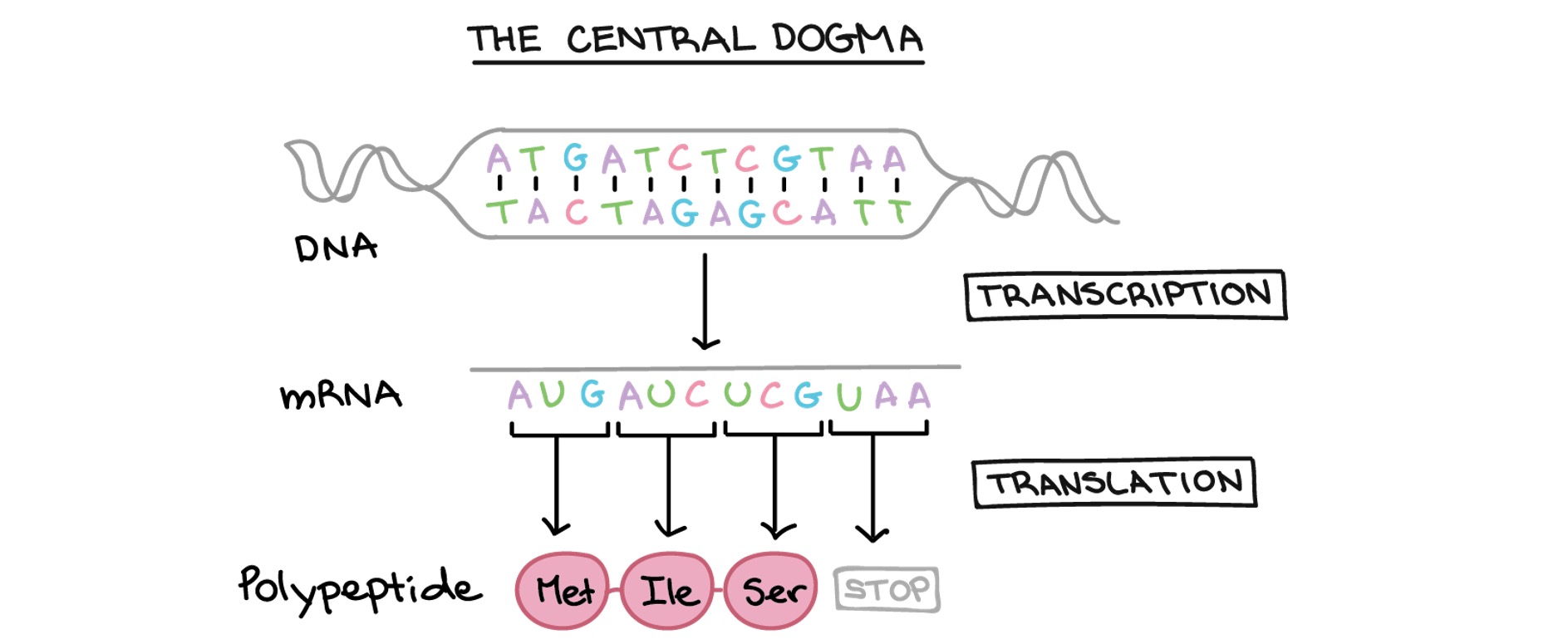
As Rett Syndrome is the result of mutations in the MeCP2 gene (DNA) that result in loss of function of the MeCP2 protein, researchers are investigating different strategies aimed to circumvent or correct the mutations, ultimately leading to expression of a functional MeCP2 protein. One of these strategies is RNA editing and a recent paper from the laboratory of Dr Gail Mandel at the Oregon Health and Science University, Portland, Oregon, USA published in the Proceeding of the National Academy of Science, USA showed that RNA editing can correct a mutation in the MeCP2 mRNA in the brain of mice with a mutation that is common in humans with Rett Syndrome.
Aims of study. The aim of the study was to induce a chemical modification in the Adenosine base in the MeCP2 mRNA by an enzyme call Adenosine Deaminase in a process termed deamination that fools the cellular machinery into thinking that the deaminated Adenosine base is a Guanosine, resulting in expression of a functional MeCP2 protein. The researchers used a mouse model which contains a Guanosine (G) to Adenosine (A) mutation at base position 311 in the MeCP2 gene (GA). This mutation generates a premature stop signal in the mRNA resulting in loss of expression of the MeCP2 protein.
Research strategy and outcome. The strategy depends on the construction of a recombinant adeno-associated virus (AAV) which delivers the gene that encodes the deaminase enzyme and a small RNA molecule (guide RNA) that recognises the mutation site in the mRNA. This results in deamination of the Adenosine base resulting in expression of functional MeCP2 protein.
The AAV was delivered in the mice by intravenous injection and because AAV can breach the blood-brain barrier that is normally impervious to circulating pathogenic microorganisms, this resulted in delivery of the AAV to neurons in the brain followed by expression of the guide RNA and the deaminase enzyme. Four weeks later, the investigators examined the efficiency of the RNA editing process by analysis of the sequence of the MeCP2 mRNA in the brain. This showed that 18% of the MeCP2 mRNA in the brainstem and 15% in the midbrain contained a G residue at base position 311, consistent with A to G editing by the recombinant AAV. A G residue at position 311 is indicative of normal MeCP2.
The researchers then examined expression of the MeCP2 protein in neurons targeted by the
AAV. Consistent with the results of the RNA editing, MeCP2 protein was detected and was associated with specific regions in the nucleus of cells that are normally associated with the function of MeCP2. By comparison with the level of MeCP2 protein in normal mice, the level of MeCP2 expression in the repaired cells from AAV treated, Rett syndrome mice was approximately 70% that of cells from control mice.
The investigators then examined the survival of the AAV-treated Rett mice and found that the life span of the treated mice increased from 11 weeks to 16 weeks. Furthermore, the AAV-treated mice showed a significant improvement in breathing irregularities, essentially, prolonged pauses in breathing that were reduced. This was not unexpected because the brainstem regulates heart and respiratory function, and the latter represents a severe symptom in Rett syndrome patients.
Discussion. Approximately 45% of mutations in MeCP2 that may be suitable for RNA editing are G to A mutations. This study shows that RNA editing can in principle correct a common mutation in the MeCP2 mRNA leading to expression of functional MeCP2 protein and amelioration of the associated symptoms. Although mRNA is short lived, new copies of MeCP2 mRNA are constantly generated (transcribed) from the MeCP2 gene (DNA). Similarly, AAV delivery of genes is thought to result in expression from the newly delivered gene for 15 years or longer, so that continuous expression of the deaminase enzyme after AAV therapy can be predicted to repair the mutation in the MeCP2 mRNA over this period.
However, there is still much work to be done before this treatment can be considered for use in patients; it is unclear what proportions of neurons should be targeted and the level of expression of the McCP2 protein required to achieve a “cure”. Nevertheless, we in the Rett community are encouraged by these results and continue to hope that these and other investigators continue to make progress towards an eventual cure.
Reference.
Sinnamon JR et al (2022). Targeted RNA editing in brainstem alleviates respiratory disfunction in a mouse model of Rett syndrome. PNAS 119; e2206053119.
Eric Gowans, Adelaide
